Highly diastereoselective synthesis of enantioenriched anti-α-allyl-β-fluoroamines†
Abstract
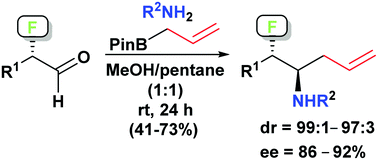
A highly diastereoselective synthesis of anti-α-allyl-β-fluoroamines has been developed involving enantioselective α-fluorination of aldehydes followed by a diastereoselective Petasis allyl borono-Mannich reaction. The products are obtained generally in good overall yields for the two steps and with drs of 97 : 3–99 : 1 and ees of 86–92%. Selected products were converted to 3-, 5- and 6-membered ring heterocycles, the latter two types incorporating an exo-cyclic fluorine.


 Please wait while we load your content...
Please wait while we load your content...