Unique Tyr-heme double cross-links in F43Y/T67R myoglobin: an artificial enzyme with a peroxidase activity comparable to that of native peroxidases†
Abstract
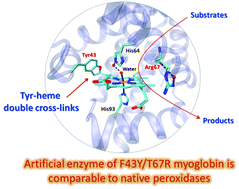
The X-ray crystal structure of F43Y/T67R myoglobin revealed unique Tyr-heme double cross-links between Tyr43 and the heme 4-vinyl group, which represents a novel post-translational modification of heme proteins. Moreover, with the feature of a distal His–Arg pair, the designed artificial enzyme exhibited a peroxidase activity comparable to that of native peroxidases, such as the most efficient horseradish peroxidase.


 Please wait while we load your content...
Please wait while we load your content...