Electrochemical 1,4-reduction of α,β-unsaturated ketones with methanol and ammonium chloride as hydrogen sources†
Abstract
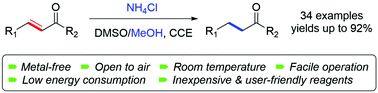
A sustainable, chemoselective 1,4-reduction of α,β-unsaturated ketones by means of an electrochemical method is presented, wherein the extremely inexpensive ammonium chloride (NH4Cl) is applied as the only additive. The reaction proceeds smoothly in the air at ambient temperature. Mechanistic studies reveal that both NH4Cl and solvent methanol work as hydrogen donors.


 Please wait while we load your content...
Please wait while we load your content...