Nickel-catalysed dehydrogenative coupling of aromatic diamines with alcohols: selective synthesis of substituted benzimidazoles and quinoxalines†
Abstract
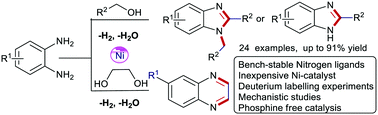
The first nickel-catalysed dehydrogenative coupling of primary alcohols and ethylene glycol with aromatic diamines for selective synthesis of mono- and di-substituted benzimidazoles and quinoxalines is reported. The earth-abundant, non-precious and simple NiCl2/L1 system enables the synthesis of N-heterocycles releasing water and hydrogen gas as byproducts. Mechanistic studies involving deuterium labeling experiments and quantitative determination of hydrogen gas evaluation were performed.
- This article is part of the themed collection: Most popular organic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...