Rhodium(ii)-catalyzed annulation of N-sulfonyl-1,2,3-triazoles with 1,3,5-triazinanes to produce octahydro-1H-purine derivatives: a combined experimental and computational study†
Abstract
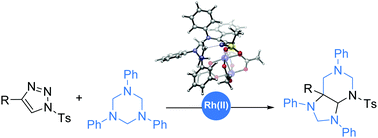
The Rh(II)-catalyzed annulation of N-sulfonyl-1,2,3-triazoles with 1,3,5-triazinanes leading to the octahydro-1H-purine derivatives with moderate to good yields is described in this communication. Mechanistic studies via DFT calculations suggest that the 1,3,5-triazinanes might undergo a formal [6+3] cycloaddition with the Rh(II)-azavinyl carbene intermediates, which are generated from Rh(II)-catalyzed denitrogenation of 1,2,3-triazoles. Afterwards, ring-closure of the formed nine-membered ring intermediate via intramolecular nucleophilic addition followed by subsequent rearrangements could afford the final octahydro-1H-purine derivatives.


 Please wait while we load your content...
Please wait while we load your content...