Iron-catalyzed oxidative C–C(vinyl) σ-bond cleavage of allylarenes to aryl aldehydes at room temperature with ambient air†
Abstract
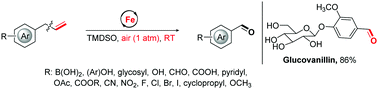
A general and selective iron-catalyzed allylic C–C(vinyl) σ-bond cleavage of allylarenes without the assistance of heteroatoms to give aryl aldehydes is reported. The unstrained carbon–carbon single bond cleavage reaction uses ambient air as the sole oxidant, proceeds efficiently at room temperature, and allows for exceptional functional-group tolerance, which addresses the long-standing challenges of current C–C bond cleavage/functionalization. Notably, the method enables rapid late-stage oxidation of complex bioactive molecules and can be used to expedite syntheses of natural products (vanillin and glucovanillin) from readily available chemical feedstocks.


 Please wait while we load your content...
Please wait while we load your content...