Synthesis of N-unsubstituted cycloalkylimines containing a 4 to 8-membered ring†
Abstract
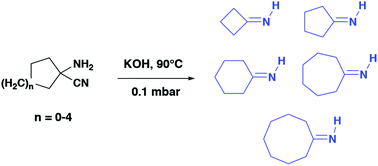
Primary cycloalkylimines with a 4 to 8-membered ring were synthesized by dehydrocyanation of the corresponding α-aminonitriles on solid potassium hydroxide via a vacuum gas–solid reaction. Imine–enamine tautomerism has been demonstrated at room temperature for the most kinetically stable derivatives.


 Please wait while we load your content...
Please wait while we load your content...