Facile o-quinodimethane formation from benzocyclobutenes triggered by the Staudinger reaction at ambient temperature†
Abstract
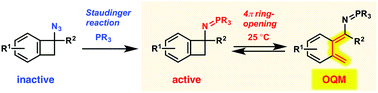
Electron-donating iminophosphoranes were found to significantly enhance 4π-ring opening of benzocyclobutenes to generate o-quinodimethanes at 20–25 °C. These iminophosphorane benzocyclobutenes can be conveniently generated from azide benzocyclobutenes and phosphines via the Staudinger reaction. Thus, Staudinger reaction-triggered sequential molecular transformations of the azide benzocyclobutenes have been established via o-quinodimethanes at ambient temperature, which is expected to exhibit potential for a wide range of applications.


 Please wait while we load your content...
Please wait while we load your content...