Finding a soft spot for vanadium: a P-bound OCP ligand†
Abstract
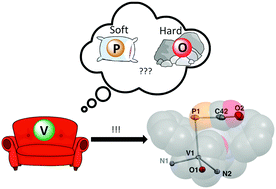
Transmetallation studies with the phosphaethynolate ion, [OCP]−, have largely resulted in coordination according to classical Lewis acid–base theory. That is, for harder early transition metal ions, O-bound coordination has been observed, whereas in the case of softer late transition metal ions, P-bound coordination predominates. Herein, we report the use of a V(III) complex, namely [(nacnac)VCl(OAr)] (1) (nacnac− = [ArNC(CH3)]2CH; Ar = 2,6-iPr2C6H3), to transmetallate [OCP]− and bind via the P-atom as [(nacnac)V(OAr)(PCO)] (2), the first example of a 3d early transition metal that binds [OCP]−via the P-atom. Full characterization studies of this molecule including HFEPR spectroscopy, SQuID measurements, and theoretical studies are presented.
- This article is part of the themed collection: Celebrating Latin American Talent in Chemistry


 Please wait while we load your content...
Please wait while we load your content...
