A consecutive process for C–C and C–N bond formation with high enantio-and diastereo-control: direct reductive amination of chiral ketones using hydrogenation catalysts†
Abstract
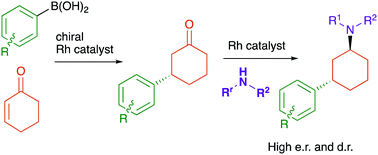
High diastereoselectivity was observed in the Rh-catalysed reductive amination of 3-arylcyclohexanones to form tertiary amines. This was incorporated into a one-pot enantioselective conjugate addition and diastereoselective reductive amination, including an example of assisted tandem catalysis.


 Please wait while we load your content...
Please wait while we load your content...