Unusual temperature-promoted carbon dioxide capture in deep-eutectic solvents: the synergistic interactions†
Abstract
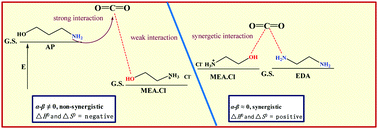
A series of novel ethylenediamine (EDA)-based deep-eutectic solvents (DESs) gave rise to unexpectedly large carbon dioxide (CO2) capture capacities at higher temperatures owing to the “synergistic interaction” between the donor and acceptor moieties.


 Please wait while we load your content...
Please wait while we load your content...