Expanding the genetic code with a lysine derivative bearing an enzymatically removable phenylacetyl group†
Abstract
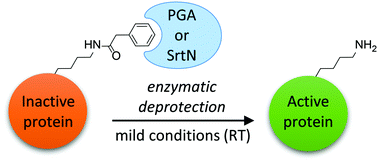
We report the genetically encoded incorporation of phenylacetyl protected lysine (PacK) into proteins in Escherichia coli. This unnatural side-chain modification can be enzymatically removed using either penicillin G acylase (PGA) or, surprisingly, the sirtuin SrtN from Bacillus subtilis. Our approach expands the toolbox to reversibly control protein structure and function under very mild and non-denaturing conditions, as demonstrated by triggering the activity of the nonribosomal peptide synthetase GrsA.


 Please wait while we load your content...
Please wait while we load your content...