Gold(i)-catalyzed enantioselective synthesis of polycyclic indoline skeletons and enantiomerically enriched β-substituted tryptamine-allenes by kinetic resolution†
Abstract
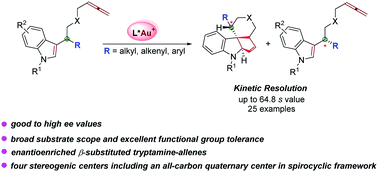
A gold(I)-catalyzed highly efficient kinetic resolution of indole–allene derivatives has been demonstrated, providing enantiomerically enriched polycyclic indolines possessing four stereogenic centers including an all-carbon quaternary center in a spirocyclic framework and β-substituted tryptamine derivatives in good to excellent ee values and high s selectivities with good functional group tolerance under mild conditions in an atom-economical way.


 Please wait while we load your content...
Please wait while we load your content...