A novel thermogel system of self-assembling peptides manipulated by enzymatic dephosphorylation†
Abstract
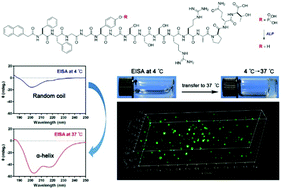
Supramolecular hydrogels of self-assembling peptides and thermogels are very promising for biomedical applications. However, there were no thermogels of self-assembling peptides. In this study, we reported on a novel and versatile strategy to prepare thermogels of self-assembling peptides by enzyme-instructed peptide folding and self-assembly. We synthesized two phosphorylated peptides from insulin growth factor (IGF) and the second mitochondria-derived activator of caspases (Smac) (Nap-FFGGpYGSSSRRAPQT and NBD-GFFpYGAVPIAQK, respectively), which could be converted to possible hydrogelators by enzyme-instructed self-assembly (EISA). We found that EISA using phosphatase at 4 °C resulted in peptides with a random coil conformation, which would self-assemble into worm-like micelles or very short fibers in clear solutions. At a physiological temperature of 37 °C, the peptides would undergo fast transitions from random coil to β-sheet- or α-helix-like conformations, resulting in solution-to-gel transformations. This novel thermogel system was very useful for three-dimensional (3D) cell culture due to the biocompatibility and bioactivity of peptides. Our study provides a novel strategy to prepare a novel thermogel system for biomedical applications.


 Please wait while we load your content...
Please wait while we load your content...