Discrepancy between thermodynamic and kinetic stabilities of the tert-butanol hydrates and its implication for obtaining pharmaceutical powders by freeze-drying†
Abstract
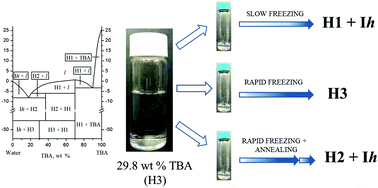
The tert-butanol (TBA)–water system is studied in relation to increasing the efficiency of obtaining pharmaceutical powders by freeze-drying. Trehalose was used as a model target product. We report the X-ray diffraction and thermal analysis data which add surprising new information to the phase diagram of this previously repeatedly studied system. The freezing protocol has a strong impact on the specific surface area of the trehalose freeze-dried cakes and on the primary drying time. This is related to a discrepancy between the kinetic and thermodynamic stabilities of several TBA hydrates: di-hydrate (H1), heptahydrate (H2), and decahydrate (H3).


 Please wait while we load your content...
Please wait while we load your content...