Photoredox-catalyzed sulfonylation of alkyl iodides, sulfur dioxide, and electron-deficient alkenes†
Abstract
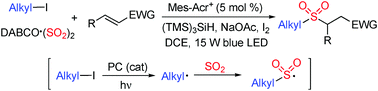
A photoredox-catalyzed sulfonylation of alkyl iodides, sulfur dioxide, and electron-deficient alkenes under mild conditions is achieved. This reaction proceeds through alkyl radicals formed in situ from alkyl iodides under visible light irradiation in the presence of a photoredox catalyst. The alkyl radical intermediates would react with sulfur dioxide leading to alkylsulfonyl radicals, which would be trapped by electron-deficient alkenes giving rise to alkyl sulfones. Various functional groups including nitro, halo, acetyl, sufonyl, and pyridinyl are all tolerated under the photoredox conditions.
- This article is part of the themed collection: Most popular organic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...