A general approach to non-fullerene electron acceptors based on the corannulene motif†
Abstract
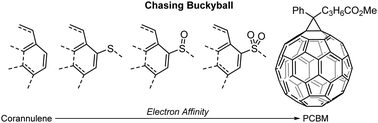
Here, we show that oxidation of exo-cyclic sulfur atoms enhances the molecular reduction potential of non-planar polycyclic aromatic hydrocarbons and allows for a systematic bridging of the electron affinity gap between corannulene, a fragment of fullerene C60, and the prevalent fullerene-based electron acceptor phenyl-C61-butyric acid methyl ester (PCBM).


 Please wait while we load your content...
Please wait while we load your content...