Acetylene-based two-step diastereoselective synthesis of bridgehead dihydro-oxadiazines using ketones and hydrazine as the only reactants†
Abstract
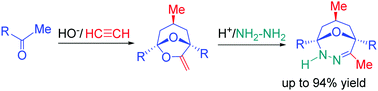
Pharmaceutically related bridgehead dihydro-1,3,4-oxadiazines are synthesized in up to 94% yield by the acid-catalyzed diastereoselective reaction of hydrazine hydrate with 6,8-dioxabicyclo[3.2.1]octanes (6,8-DOBCOs), the products of the superbase-promoted self-organization of acetylene with ketones. The synthesis covers a diverse range of alkylaromatic ketones having F-, Cl-, Br-, alkoxy-, CF3- and aryl substituents in different positions of the aromatic rings.


 Please wait while we load your content...
Please wait while we load your content...