Nickel-catalyzed alkyl–alkyl cross-coupling reactions of non-activated secondary alkyl bromides with aldehydes as alkyl carbanion equivalents†
Abstract
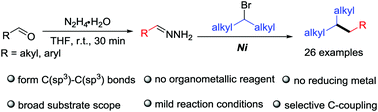
A novel nickel-catalyzed alkyl–alkyl cross coupling of non-activated secondary alkyl bromides with aldehydes via hydrazone intermediates has been developed. Aldehydes as alkyl carbanion equivalents replace traditional organometallic reagents. This coupling occurs on the carbon of the hydrazone rather than the nitrogen. In addition, non-activated primary and tertiary alkyl bromides also undergo the cross-coupling reaction to form new C(sp3)–C(sp3) bonds in moderate yields.


 Please wait while we load your content...
Please wait while we load your content...