Intermolecular, redox-neutral azidoarylation of alkenes via photoredox catalysis†
Abstract
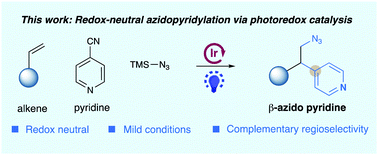
An intermolecular, redox-neutral azidoarylation of alkenes with pyridines and TMSN3 has been reported via visible light-induced photoredox catalysis. This protocol utilized a radical addition/radical coupling sequence, allowing for facile and regioselective installation of versatile β-azido pyridines under redox-neutral and mild conditions.


 Please wait while we load your content...
Please wait while we load your content...