Rapid nickel(ii)-promoted cysteine S-arylation with arylboronic acids†
Abstract
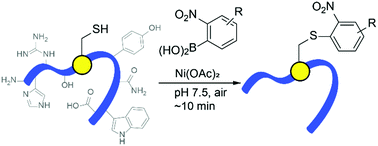
S-Arylation of cysteine residues is an increasingly powerful tool for site-specific modification of proteins, providing novel structure and electronic perturbation. The present work demonstrates an operationally-simple cysteine arylation reaction 2-nitro-substituted arylboronic acids, promoted by a simple nickel(II) salt. The process exhibits strikingly fast reaction rates under physiological conditions in purely aqueous media with excellent selectivity toward cysteine residues. Cysteine arylation of natural proteins and peptides allows attachment of useful reactive handles for stapling, imaging, or further conjugation.


 Please wait while we load your content...
Please wait while we load your content...