Visible-light activated metal catalyst-free vicinal diazidation of olefins with sulfonium iodate(i) species†
Abstract
A visible light-induced vicinal diazidation of various alkenes using stable sulfonium bis(acetoxy)iodate(I) and sodium azide as a radical precursor is described. The trimethylsulfonium [bis(azido)iodate(I)] species, intrinsically generated in situ, demonstrated unprecedented reactivity toward C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C π bond-promoting selective azidation under photochemical conditions without any photoredox or metal catalyst. The visible-light stimulated diazidation reaction provides a convenient and straightforward approach to highly prevalent vicinal nitrogen scaffolds of myriad therapeutic importance.
C π bond-promoting selective azidation under photochemical conditions without any photoredox or metal catalyst. The visible-light stimulated diazidation reaction provides a convenient and straightforward approach to highly prevalent vicinal nitrogen scaffolds of myriad therapeutic importance.
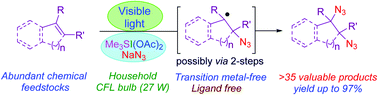
- This article is part of the themed collection: 20th Anniversary of the CRSI: Celebrating Indian Chemistry in ChemComm


 Please wait while we load your content...
Please wait while we load your content...