Electrodeposition of indium from non-aqueous electrolytes†
Abstract
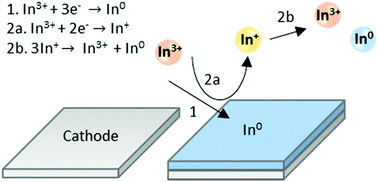
The electrochemical behaviour and deposition of indium in electrolytes composed of 0.4 mol dm−3 In(Tf2N)3 and 0.4 mol dm−3 InCl3 in the solvents 1,2-dimethoxyethane and poly(ethylene glycol) (average molecular mass of 0.400 kg mol−1, PEG400) was investigated. Indium(I) was identified as the intermediate species that disproportionated to indium(III) and indium(0) nanoparticles. The presence of nanoparticles was verified by TEM analysis. SEM analysis showed that deposits obtained at room temperature from 1,2-dimethoxyethane were rough, while spherical structures were formed in PEG400 at 160 °C.


 Please wait while we load your content...
Please wait while we load your content...