Palladium-catalyzed cross-coupling of unactivated alkylzinc reagents with 2-bromo-3,3,3-trifluoropropene and its application in the synthesis of fluorinated amino acids†
Abstract
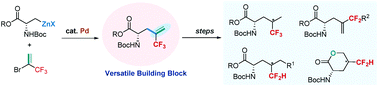
A palladium-catalyzed cross-coupling of unactivated alkylzinc reagents with 2-bromo-3,3,3-trifluoropropene (BTP) has been developed, which was used as a key step to prepare a series of trifluoromethylated and difluoromethylated amino acids that are of great interest in peptide/protein based chemical biology. The advantages of the synthesis of these fluorinated amino acids are synthetic simplicity and diversity from a simple and readily available key intermediate α-trifluoromethylalkene-containing amino acid, providing a facile route for applications in medicinal chemistry and life science.


 Please wait while we load your content...
Please wait while we load your content...