Radical-induced ring-opening and reconstruction of cyclobutanone oxime esters†
Abstract
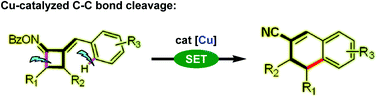
The first example of intramolecular ring-opening and reconstruction of cyclobutanone oxime esters via selective C–C bond cleavage leading to the synthesis of 3,4-dihydronaphthalene-2-carbonitriles in the presence of a cheap copper catalyst has been reported. The protocol is distinguished by mild and safe reaction conditions that exclude ligands, oxidants, bases, toxic cyanide salts and tolerates a wide scope of cyclobutanones without compromising their efficiency and scalability. The alternative visible-light-driven photoredox process for this coupling reaction was also uncovered.


 Please wait while we load your content...
Please wait while we load your content...