Palladium-catalyzed N1-selective allylation of indoles with allylic alcohols promoted by titanium tetraisopropoxide†
Abstract
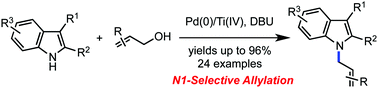
The direct N1-selective allylation of indoles with allylic alcohols has been accomplished by synergistic functions of palladium catalysts and titanium tetraisopropoxide. The site selectivity is notably different from that observed in other related transition metal-catalyzed approaches. This chemistry provides a facile route to a variety of allylated indoles in synthetically useful yields. The utility of this simple allylation reaction was demonstrated with the first total synthesis of (+)-N-(4′-hydroxyprenyl)-cyclo(alanyltryptophyl), which was completed in five steps, starting from L-tryptophan methyl ester hydrochloride.


 Please wait while we load your content...
Please wait while we load your content...