Sterol A-ring plasticity in hedgehog protein cholesterolysis supports a primitive substrate selectivity mechanism†
Abstract
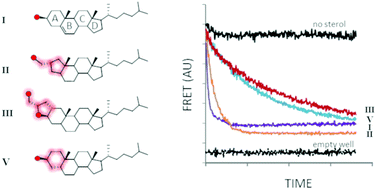
Cholesterolysis of Hedgehog family proteins couples endoproteolysis to protein C-terminal sterylation. The transformation is self-catalyzed by HhC, a partially characterized enzymatic domain found in precursor forms of Hedgehog. Here we explore spatial ambiguity in sterol recognition by HhC, using a trio of derivatives where the sterol A-ring is contracted, fused, or distorted. Sterylation assays indicate that these geometric variants react as substrates with relative activity: cholesterol, 1.000 > A-ring contracted, 0.100 > A-ring fused, 0.020 > A-ring distorted, 0.005. Experimental results and computational sterol docking into the first HhC homology model suggest a partially unstructured binding site with substrate recognition governed in large part by hydrophobic interactions.


 Please wait while we load your content...
Please wait while we load your content...