Isolation of a stable pyridine radical anion†
Abstract
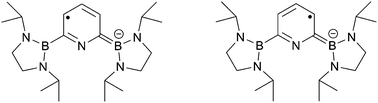
Reduction of 2,6-bis(diazaboryl)pyridine with KC8 gives a room-temperature-stable yellow colored solution containing the corresponding radical anion. The radical was characterized by single crystal XRD, EPR spectroscopy, UV-vis absorption spectroscopy and electrochemically, supported by theoretical calculations. The negative charge and spin density are mainly distributed over the atoms of the pyridine ring, making this the first isolated pyridine radical anion as its potassium salt.


 Please wait while we load your content...
Please wait while we load your content...