Near-infrared absorption by intramolecular charge-transfer transition in 5,10,15,20-tetra(N-carbazolyl)porphyrin through protonation†
Abstract
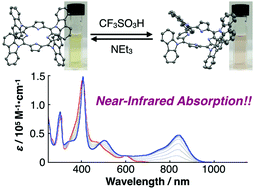
A porphyrin coupled quadruply with N-carbazolyl groups at the meso positions has been synthesized. Because of the electron-withdrawing nature of the carbazole units at the porphyrin centre, the tetra(N-carbazolyl)porphyrin and the protonated derivative display unique absorption bands derived from intramolecular charge-transfer transition from the carbazoles to the porphyrin moiety.


 Please wait while we load your content...
Please wait while we load your content...