A cobalt arylphosphonate MOF – superior stability, sorption and magnetism†
Abstract
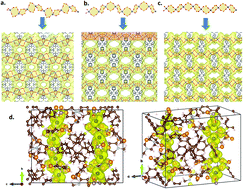
We report a novel metal-organic framework (MOF) based on a cobalt arylphosphonate, namely, [Co2(H4-MTPPA)]·3NMP·H2O (1·3NMP·H2O), which was prepared solvothermically from the tetrahedral linker tetraphenylmethane tetrakis-4-phosphonic acid (H8-MTPPA) and CoSO4·7H2O in N-methyl-2-pyrrolidone (NMP). Compound 1 has the highest porosity (BET surface area of 1034 m2 g−1) ever reported for a MOF based on an aryl phosphonic acid linker. The indigo blue crystals of 1·3NMP·H2O are composed of edge-shared eight-membered Co2P2O4 rings, and are thermally very stable up to 500 °C.


 Please wait while we load your content...
Please wait while we load your content...