Iron-catalysed enantioselective Suzuki–Miyaura coupling of racemic alkyl bromides†
Abstract
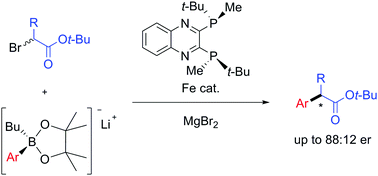
The first iron-catalysed enantioselective Suzuki–Miyaura coupling reaction has been developed. In the presence of catalytic amounts of FeCl2 and (R,R)-QuinoxP*, lithium arylborates are cross-coupled with tert-butyl α-bromopropionate in an enantioconvergent manner, enabling facile access to various optically active α-arylpropionic acids including several nonsteroidal anti-inflammatory drugs (NSAIDs) of commercial importance. (R,R)-QuinoxP* is specifically able to induce chirality when compared to analogous P-chiral ligands that give racemic products, highlighting the critical importance of transmetalation in the present asymmetric cross-coupling system.


 Please wait while we load your content...
Please wait while we load your content...