Double asymmetric hydrogenation of conjugated dienes: a self-breeding chirality route for C2 symmetric 1,4-diols†
Abstract
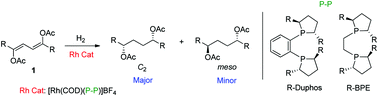
The synthesis and double asymmetric hydrogenation of (Z,Z)-1,3-diene-1,4-diyl diacetates is described. In this reaction C2/meso ratios up to 85 : 15 and enantioselectivities up to 97% ee have been achieved. As the hydrogenation products can be converted into chiral 1,4-diols, key starting materials for the preparation of the best catalysts used, this catalytic system enables a self-breeding chirality process.


 Please wait while we load your content...
Please wait while we load your content...