A ring-shaped hemoprotein trimer thermodynamically controlled by the supramolecular heme–heme pocket interaction†
Abstract
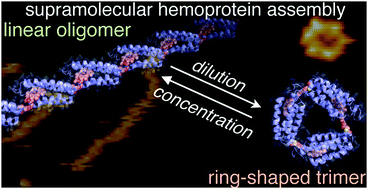
Engineered cytochrome b562, a small hemoprotein, with an externally-attached heme moiety via a moderately long linker at a suitable position predominantly forms a thermodynamically stable ring-shaped trimer in dilute solution. In an equilibrium between supramolecular polymerization and depolymerization, the ring-shaped trimer is kinetically trapped even in a concentrated solution.


 Please wait while we load your content...
Please wait while we load your content...
