Chemical and mechanistic analysis of photodynamic inhibition of Alzheimer's β-amyloid aggregation†
Abstract
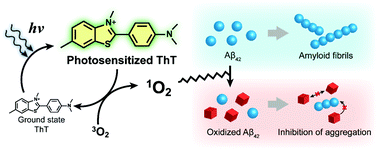
The self-assembly of the beta-amyloid peptide (Aβ) into amyloid aggregates is a central phenomenon associated with Alzheimer's disease. Here, we report chemical modifications of key amino acid residues of Aβ42 (Y10, H13, H14, and M35) by photoexcited thioflavin-T (ThT), a fluorescent probe of amyloid structure. The quantitative chemical kinetics analysis shows that the oxidized monomer species does not self-assemble, nor perturb the aggregation kinetics of non-oxidized Aβ42.


 Please wait while we load your content...
Please wait while we load your content...