Electrochemical nitrogen reduction to ammonia at ambient conditions on nitrogen and phosphorus co-doped porous carbon†
Abstract
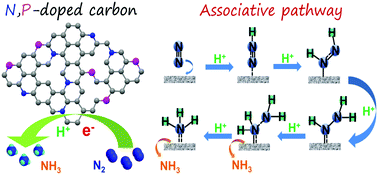
Reduction of nitrogen (N2) under ambient conditions and understanding the mechanism have been hugely challenging problems for decades. Herein, we for the first time report that N,P co-doped hierarchical porous carbon (NPC) can serve as an electrocatalyst for the nitrogen reduction reaction (NRR) in an acid aqueous solution under ambient conditions. The faradaic efficiency (FE) and yield of production of NH3 on the NPC electrode reached as high as 4.2% and 0.97 μg h−1 mg-1cat., respectively. Furthermore, the electrocatalytic NRR mechanism on the NPC electrode was undoubtedly confirmed by electrochemical in situ Fourier transform infrared spectroscopy, and follows an associative pathway. These results are predicted to offer a new platform in the rational design and synthesis of highly efficient electrocatalysts for the NRR under ambient conditions.


 Please wait while we load your content...
Please wait while we load your content...