Thermally activated delayed fluorescence and room-temperature phosphorescence in naphthyl appended carbazole–quinoline conjugates, and their mechanical regulation†
Abstract
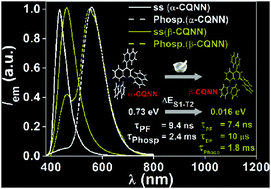
The influences of naphthyl and/or phenyl rings at the 2,4-positions of the quinolinyl fragments in carbazole–quinoline conjugates are studied. The centric phase of one of the conjugates (β-CQNN) revealed both thermally activated delayed fluorescence (TADF) and room-temperature phosphorescence (RTP), while prompt fluorescence and RTP were observed in the non-centric phase (α-CQNN) that can regenerate the emission features of β-CQNNvia mechanical grinding. This unique observation is explained by the modulation of the higher-lying triplet (T2) energy level caused by conformational change.


 Please wait while we load your content...
Please wait while we load your content...