A vinylogous Michael addition-triggered quadruple cascade reaction for the enantioselective generation of multiple quaternary stereocenters†
Abstract
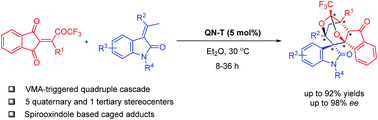
An efficient organocatalytic quadruple cascade reaction resulting in spiroxindole scaffolds bearing five quaternary stereocenters is reported. The complex cascade reaction is triggered by the scarcely explored vinylogous Michael addition of 3-alkylidene oxindoles to fully substituted enones and demonstrates the usefulness of the latter as efficient Michael acceptors in generating complex caged products in 26–92% yields, 14–98% ee and up to >25 : 1 d.r. values.


 Please wait while we load your content...
Please wait while we load your content...