Tuning the reduction potential of quinones by controlling the effects of hydrogen bonding, protonation and proton-coupled electron transfer reactions†
Abstract
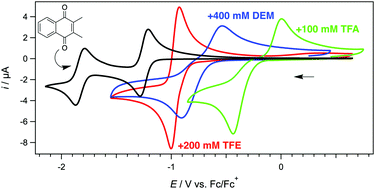
An all-organic cell comprising 2,3-dimethyl-1,4-napthoquinone and pyrano[3,2-f]chromene as electroactive elements exhibited a good combination of large cell voltage and stability of the reduced quinone upon the addition of diethyl malonate (a weak organic acid), as compared to the addition of trifluoroethanol (which led to a high cell potential but low stability via strong hydrogen bonding interactions) and the addition of trifluoroacetic acid (which led to a lower cell potential but high stability through proton transfer).
- This article is part of the themed collection: Frontiers in Proton Coupled Electron Transfer (PCET)


 Please wait while we load your content...
Please wait while we load your content...