Palladium-catalyzed trisallylation of benzoxazoles and 2-aryl-1,3,4-oxadiazoles with alkyne†
Abstract
A Pd-catalyzed direct C–H trisallylation of azoles with alkyne has been described. A consecutive C(sp2)–H allylation/olefin isomerization/double C(sp3)–H allylation of azoles and alkyne via a palladium catalysis system proceeds efficiently to afford the corresponding C2-alkenylated azoles containing an all quaternary carbon center.
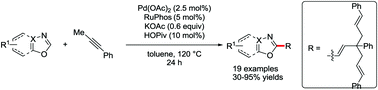


 Please wait while we load your content...
Please wait while we load your content...