Copper-catalyzed aminothiolation of terminal alkynes with tunable regioselectivity†
Abstract
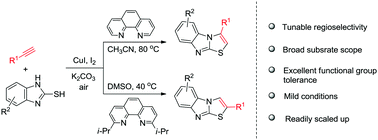
A simple, mild, and efficient catalytic aminothiolation of terminal alkynes for the synthesis of both 2- and 3-substituted thiazolo[3,2-a]benzimidazoles is established upon catalysis with copper(I), in which complementary regioselectivities could be achieved by using sterically different phenanthroline-based ligands.


 Please wait while we load your content...
Please wait while we load your content...