On water N-arylation of oxetanylamines for the preparation of N-aryl-oxetanylamines; potentially useful aryl-amide isosteres†
Abstract
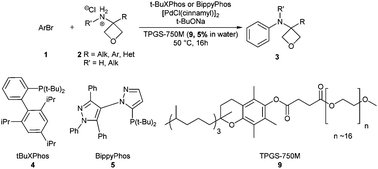
A Pd cross-coupling approach for the synthesis of N-aryl-oxetanylamines has been developed. This method provides new building blocks potentially useful in medicinal chemistry as amide bioisosteres. The reactions are conducted in water employing the renewable feedstock surfactant TPGS-750-M.


 Please wait while we load your content...
Please wait while we load your content...