Chiral iminophosphorane catalyzed asymmetric sulfenylation of 4-substituted pyrazolones†
Abstract
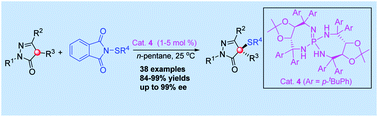
An excellent level of enantioselectivity in asymmetric sulfenylation of 4-substituted pyrazolones was achieved with chiral iminophosphorane as the organocatalyst under continuum solvation conditions (up to 99% ee). Importantly, this catalytic process features high efficiency with excellent enantioselectivities, easy separation of products, low catalytic loadings and scale-up to grams without loss of enantioselectivity.


 Please wait while we load your content...
Please wait while we load your content...