A self-hydrosilylation of phosphanylhydrosilylalkynes promoted by B(C6F5)3? An experimental and mechanistic study†
Abstract
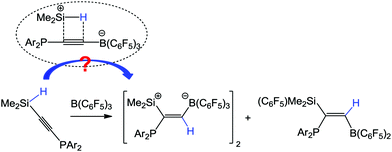
Phosphanylhydrosilylalkynes Me2HSiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPAr2 (Ar = Ph, 1a; 4-MeC6H4, 1b) were synthesized, which reacted with B(C6F5)3 to produce alkenes [(E)-(C6F5)3BCH
CPAr2 (Ar = Ph, 1a; 4-MeC6H4, 1b) were synthesized, which reacted with B(C6F5)3 to produce alkenes [(E)-(C6F5)3BCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(PAr2)SiMe2]2 (2a and 2b) and (Z)-(C6F5)2BCH
C(PAr2)SiMe2]2 (2a and 2b) and (Z)-(C6F5)2BCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(PAr2)SiMe2(C6F5) (3a and 3b). The formation of 2a (or 2b) involved a Wrackmeyer's SiHMe2 migration followed by Si–H addition across the C
C(PAr2)SiMe2(C6F5) (3a and 3b). The formation of 2a (or 2b) involved a Wrackmeyer's SiHMe2 migration followed by Si–H addition across the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond, whereas, that of 3a (or 3b) involved a similar mechanism with a further C6F5 migration. The B(C6F5)3-promoted reaction of the Si-centered geminal H and C
C bond, whereas, that of 3a (or 3b) involved a similar mechanism with a further C6F5 migration. The B(C6F5)3-promoted reaction of the Si-centered geminal H and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C groups is thus realized, which may be considered as a self-hydrosilylation. Mechanistic studies by both variable temperature NMR spectroscopy and DFT calculations were accomplished.
C groups is thus realized, which may be considered as a self-hydrosilylation. Mechanistic studies by both variable temperature NMR spectroscopy and DFT calculations were accomplished.
- This article is part of the themed collection: Celebrating a Century of Excellency in Chemistry at Xiamen University


 Please wait while we load your content...
Please wait while we load your content...