Improved synthesis of β-ketoenamine-linked covalent organic frameworks via monomer exchange reactions†
Abstract
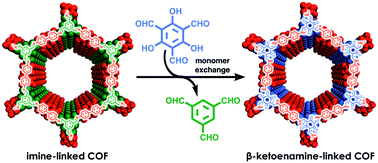
β-Ketoenamine-linked covalent organic frameworks (COFs) offer excellent structural versatility and outstanding aqueous stability, but their stability complicates obtaining samples with high crystallinity and surface areas. In contrast, imine-linked COFs are often isolated with superior materials quality. Here we synthesize several β-ketoenamine-linked COFs, including two unreported structures, with unmatched crystallinity and high surface areas by preparing the corresponding imine-linked COF and exchanging its triformylbenzene monomers with triformylphloroglucinol.


 Please wait while we load your content...
Please wait while we load your content...