Hydrogen bonding promoted simple and clean photo-induced reduction of C–X bond with isopropanol†
Abstract
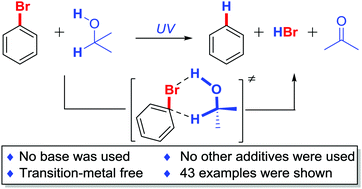
We herein report a simple and clean photo-induced metal-free reduction of C–X bond under an atmosphere of air at room temperature. Isopropanol is used as both the reducing reagent and solvent. Various functional groups (acids, esters, alcohols, anilines, phenols, indoles, pyridines, cyano and trifluoromethyl groups) and other heterocyclic compounds are tolerated. Different organic halides (including C–I, C–Br and C–Cl bonds) can be dehalogenated with moderate to excellent yields. Polyhalides are also reduced chemoselectively and efficiently. DFT calculation suggests a six-membered ring transition state via C–X⋯H–O hydrogen bonding to decrease the activation energy.


 Please wait while we load your content...
Please wait while we load your content...