Stereogenic cyclic oligonaphthalenes displaying ring size-dependent handedness of circularly polarized luminescence (CPL)†
Abstract
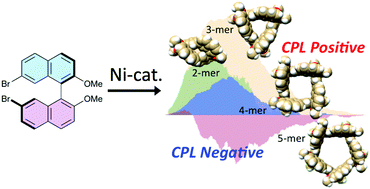
A series of cyclic oligomers with Dn symmetry (n = 2–5) based on chiral (R)/(S)-binaphthyl exhibiting intensive chiroptical properties were synthesized by using a Ni(0)-catalyzed coupling reaction. The intensity and the handedness of circularly polarized luminescence (CPL) were dependent on the ring size of the cyclic oligomers, in which the 1,1′-binaphthyl moieties form s-cis (n = 2, 3) or s-trans (n = 4, 5) conformers in the excited state. The relationship between the structure and chiroptical properties is discussed with the aid of X-ray analysis and DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...