Solid-phase synthesis for thalidomide-based proteolysis-targeting chimeras (PROTAC)†
Abstract
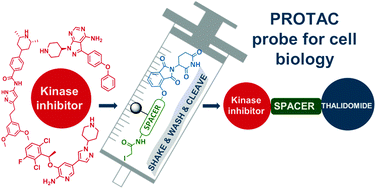
A preloaded resin consisting of a thalidomide moiety and an ethylene-oxy linker allows the simple and fast formation of PROTACs. The feasibility of the procedure was illustrated by conjugating different protein kinase inhibitors. The biological functionality of an ibrutinib-like conjugate was then confirmed by a cellular experiment.


 Please wait while we load your content...
Please wait while we load your content...