Versatile approach to activation of alkoxyamine homolysis by 1,3-dipolar cycloaddition for efficient and safe nitroxide mediated polymerization†
Abstract
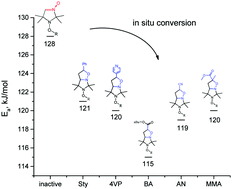
An alkoxyamine was prepared from a cyclic aldonitrone nitroxide. The resulting alkoxyamine containing an aldonitrone functional substituent is relatively stable but can react readily with vinyl monomers to form a cycloadduct that has a much higher C–ON homolysis rate. This type of in situ activation converts the aldonitrone alkoxyamine into an efficient controlling agent for nitroxide-mediated polymerization. Here we present a study on this reaction of C–ON bond homolysis and application of such an alkoxyamine as an in situ-activated initiator.


 Please wait while we load your content...
Please wait while we load your content...