One-pot, modular approach to functionalized ketones via nucleophilic addition/Buchwald–Hartwig amination strategy†
Abstract
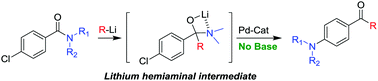
A general one-pot procedure for the 1,2-addition of organolithium reagents to amides followed by the Buchwald–Hartwig amination with in situ released lithium amides is presented. In this work amides are used as masked ketones, revealed by the addition of organolithium reagents which generates a lithium amide, suitable for subsequent Buchwald–Hartwig coupling in the presence of a palladium catalyst. This methodology allows for rapid, efficient and atom economic synthesis of aminoarylketones in good yields.


 Please wait while we load your content...
Please wait while we load your content...