Alkenylation of unactivated alkyl bromides through visible light photocatalysis†
Abstract
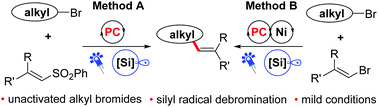
Two visible-light driven alkenylation reactions of unactivated alkyl bromides, which were enabled by the use of Ir(dF(CF3)ppy)2(dtbbpy)PF6 as the photocatalyst and (TMS)3SiH as the atom transfer reagent to activate the alkyl bromides, were described for the first time. These protocols can be used to produce a variety of alkenes from easily available feedstock with good reaction efficiency and high chemoselectivity under mild reaction conditions. To further demonstrate the applicability of the present strategy, the alkenylation of bioactive molecules and glycosyl bromides, as well as the alkynylation of unactivated alkyl bromides, was proven to be feasible.


 Please wait while we load your content...
Please wait while we load your content...